Precision in injection molding design plays a pivotal role in medical device manufacturing. The intricate molding process demands exactness to ensure parts meet stringent standards. Failure to maintain tight tolerances can lead to defects that compromise both quality and functionality. Tools like CAD software and injection molding process control systems help designers achieve the required precision. Statistical methods, including tolerance analysis and control charts, provide insights into critical factors affecting product quality. Process capability studies evaluate how well manufacturing processes align with specifications, ensuring reliable outcomes during production. By prioritizing quality control and inspection, manufacturers can meet regulatory requirements and deliver dependable devices.
Selecting the right material is critical for ensuring the quality and functionality of injection molded medical devices. Medical-grade materials must meet stringent guidelines for safety and performance. For example, improper temperature control during the mixing of PVC can lead to discoloration and cracking, compromising the material's strength. Adjusting the process can create a more homogeneous and durable material. Similarly, integrating pigments during the injection molding process instead of a separate step can prevent molecular weight reduction, which often causes cracks after aging. These examples highlight the importance of precise material selection and process control in achieving high-quality outcomes.
Medical devices must balance biocompatibility with durability to ensure patient safety and long-term effectiveness. Materials used in injection molding must comply with biocompatibility standards while maintaining the mechanical properties necessary for their intended use. A 2018 report by the World Health Organization emphasizes that medical devices are used by up to 10% of the global population, underscoring the need for safe materials. Additionally, a 2017 study in the Journal of Clinical Medicine highlights that some implants remain in the body for over a decade, making durability a critical factor. This balance ensures that devices perform reliably without compromising patient health.
Long-term performance depends on several material properties, including creep resistance, thermal stability, and environmental stress cracking (ESC). Materials like polyethylene (PE) and polypropylene (PP) exhibit low creep due to their tightly packed molecular chains, making them ideal for applications requiring dimensional stability. High-performance thermoplastics like PEEK offer excellent thermal stability and resistance to deformation under continuous load, thanks to their strong intermolecular bonds. However, temperature changes can significantly impact polymers. According to the Arrhenius Equation, degradation rates double with every 10°C increase, emphasizing the need for materials with high thermal stability. By considering these factors, designers can ensure the durability and reliability of injection molded parts.
Uniform wall thickness plays a critical role in the injection molding process. It ensures even material flow during filling and consistent cooling, which directly impacts the quality of the final product. Variations in thickness can lead to defects such as sinks, voids, and warping. For optimal results, designers should aim to maintain wall thickness variations within 10% of the nominal value. This approach minimizes internal stresses and ensures predictable material behavior during the molding process.
| Evidence Description | Key Points |
|---|---|
| Uniform wall thickness ensures even filling and cooling. | Modifying geometry to eliminate thick sections is preferred over special cooling designs. |
| Thicker wall sections lead to longer cycle times. | The thickest part controls the cycle time if cooling is even. |
| Thicker sections fill first and shrink more, causing defects. | This can result in sinks, voids, and warpage in the final product. |
| Gradual transition from thickest to thinnest sections is essential. | This helps maintain quality and reduces defects during the molding process. |
| Consistent wall thickness prevents warping and internal stresses. | Variations should be within 10% of nominal thickness to ensure predictable material flow. |
Non-uniform wall thickness often leads to uneven cooling rates, which can cause differential shrinkage. This phenomenon results in warping, a defect that compromises the quality and functionality of injection molded parts. To prevent this issue, designers should prioritize uniform wall thickness throughout the part. Gradual transitions between thick and thin sections further reduce the risk of defects. Studies show that maintaining thickness variations within ±10% tolerance significantly minimizes warping and ensures better control over the manufacturing process.
Uniform wall thickness not only improves quality but also enhances the strength and functionality of injection molded parts. Experimental results confirm that consistent thickness reduces internal stress and ensures even strain distribution. For instance, seamless gas cylinders with uniform wall thickness exhibit consistent strain across different zones, leading to improved durability. Additionally, reduced internal stress contributes to the overall reliability of the part, making it suitable for demanding applications. By focusing on uniformity, designers can achieve superior performance and long-term reliability in their injection molding projects.
Tight tolerances are essential in injection molding for medical devices. Dimensional accuracy ensures parts meet critical functional areas and maintain consistent quality. Even minor deviations can lead to product failure, compromising patient safety. Manufacturers prioritize tolerances to align with stringent medical standards.
| Source | Key Insight |
|---|---|
| Crescent Industries | Tight tolerances are crucial in manufacturing complex parts for medical use. |
| Intertech Plastics | Dimensional accuracy ensures high-quality patient care. |
| Xometry | Proper tolerances prevent underperformance or failure of molded parts. |
Designers must evaluate tolerances during the molding process to ensure parts meet regulatory requirements. This approach minimizes defects and enhances the reliability of medical devices.
Precision tools play a vital role in achieving high-quality mold design. Injection speed and pressure are critical parameters that control material flow and filling uniformity. Designers optimize these factors to prevent defects like flash or incomplete filling.
Advanced measurement tools further enhance precision. For example, surface accuracy metrics like P-V (2.27 μm) and RMS (0.375 μm) ensure smooth finishes. A coefficient of determination (r² = 0.99) confirms the reliability of these measurements. By leveraging these tools, designers achieve consistent quality in injection molding.
Maintaining consistency in injection molding is challenging due to variations in material properties and process conditions. Designers address these challenges by implementing robust control systems. These systems monitor injection speed, pressure, and cooling rates to ensure uniformity.
Gradual adjustments to process parameters prevent defects and improve part quality. For instance, balancing injection pressure reduces the risk of flash while ensuring complete filling. Temperature control minimizes shrinkage and warping, enhancing the durability of molded parts. By focusing on consistency, manufacturers deliver reliable products that meet medical standards.
Choosing the appropriate gate type is essential for achieving high-quality injection molded parts. Different gate types, such as edge gates, tunnel gates, and center gates, offer unique advantages depending on the part design. For instance, center gate placement ensures balanced material flow, reducing warpage and shrinkage. On the other hand, gates positioned away from thicker sections improve packing efficiency and minimize voids, enhancing weld line strength. A well-designed gate facilitates smooth material flow, reducing stress and defects like underfilling or distortion. Proper gate selection directly impacts the molding process, ensuring consistent quality and tighter tolerances.
Optimized gate placement plays a pivotal role in the injection molding process. Mold flow analysis helps identify the best gate locations, ensuring uniform filling and minimizing defects. Strategic placement reduces air entrapment and enhances flow efficiency, leading to fewer scrap parts. For example, placing gates near critical functional areas ensures complete filling while maintaining part integrity. Proper coordination between gate placement and mold design improves the overall manufacturing process. This approach not only enhances the quality of injection molded parts but also reduces cycle times, improving production efficiency.
Proper gate design is crucial for controlling the filling pattern and reducing defects in injection molded parts. Strategic placement ensures uniform material flow, minimizing warpage and sink marks. For instance, edge gates can be used to eliminate undercuts in complex designs, while tunnel gates are effective for reducing visible gate marks. By ensuring even filling of the mold, designers can prevent defects like voids and distortion. Additionally, optimized gate design enhances the control over the molding process, resulting in parts with superior quality and durability. Manufacturers who prioritize gate design achieve better outcomes and reduce production waste.

Efficient cooling channels are essential for maintaining the quality of injection molded parts. Properly designed channels ensure uniform heat dissipation, which prevents defects like warping and residual stress. Advanced tools such as thermographic cameras and pressure/flow meters enable real-time monitoring of surface temperature and flow performance. These tools validate the effectiveness of cooling channel designs by comparing simulation results with actual test data. For instance:
- Simulations closely align with experimental results, confirming the accuracy of conformal cooling channels.
- A temperature difference of only 1.3°C between simulation and experiment highlights the precision of these designs.
By leveraging these technologies, manufacturers can optimize cooling systems to enhance the injection molding process.
Efficient cooling systems significantly reduce cycle times while maintaining product quality. Shorter cycle times improve production efficiency and lower costs without compromising the integrity of molded parts. The following statistics illustrate the impact of optimized cooling systems:
| Statistic | Value |
|---|---|
| Cycle time reduction | 20-30% |
| Scrap rate reduction | 15% |
| Energy consumption reduction | 10-25% |
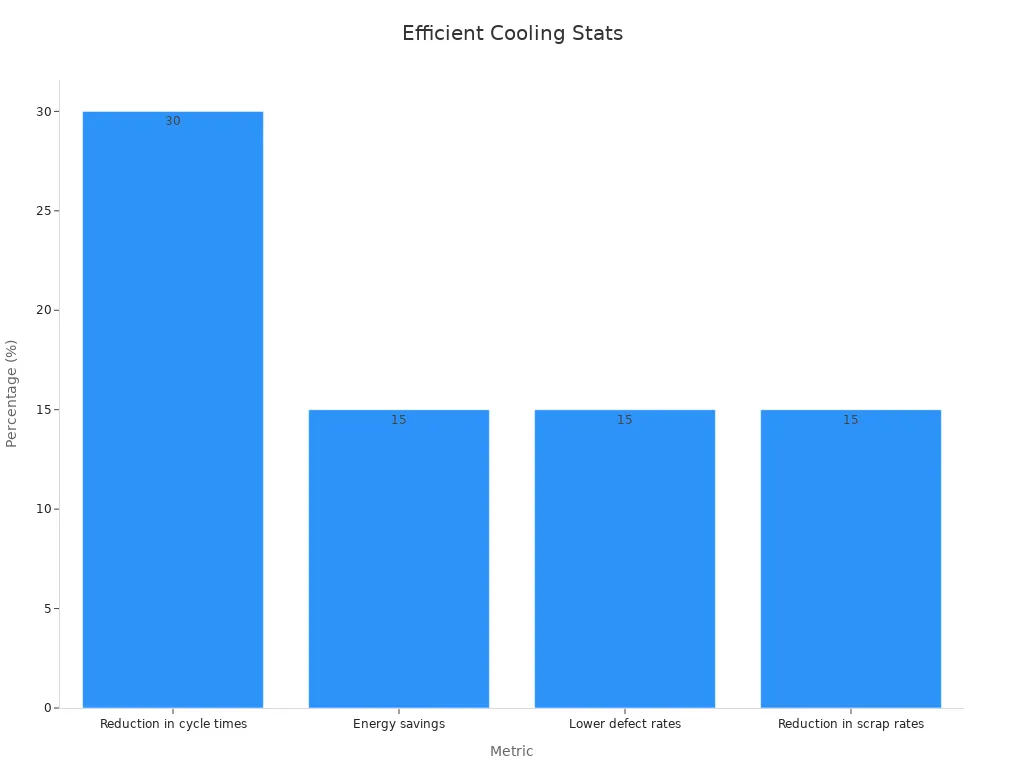
These improvements not only enhance process stability but also increase throughput and reduce labor costs per unit. By focusing on cooling efficiency, manufacturers achieve higher productivity and better control over the injection molding process.
Uniform cooling is critical for ensuring the stability and consistency of injection molded parts. Uneven cooling can create thermal gradients, leading to defects such as warpage and residual stress. Proper cooling strategies eliminate these issues, preserving the mechanical integrity of the material. The following table highlights the benefits of uniform cooling:
| Evidence | Explanation |
|---|---|
| Prevents thermal gradients | Reduces defects like warpage and residual stress. |
| Ensures consistent dimensional stability | Maintains the intended shape and size of the product. |
| Preserves mechanical integrity | Enhances strength and durability, reducing the risk of failure. |
By prioritizing uniform cooling, designers can improve the quality and reliability of injection molded parts, ensuring they meet stringent manufacturing standards.

Smooth surface finishes are critical for medical devices, as they directly impact patient safety and device functionality. A polished surface minimizes the risk of bacterial growth and ensures compatibility with sterilization methods. Designers achieve smooth finishes by carefully selecting mold materials and applying advanced polishing techniques. For instance, using hardened steel molds with a mirror-like finish reduces surface imperfections during the injection process. Additionally, maintaining precise temperature control during molding prevents defects like flow lines, which can compromise surface quality. By prioritizing smooth finishes, manufacturers enhance the reliability and safety of medical devices.
Surface texture plays a vital role in the performance of injection molded parts. Textured surfaces improve grip, reduce glare, and enhance the aesthetic appeal of medical devices. In some cases, textures also serve functional purposes, such as reducing friction or aiding in fluid flow. For example, micro-textures on catheter surfaces minimize resistance during insertion, improving patient comfort. Designers must balance texture depth and uniformity to ensure consistent quality across all parts. Properly controlled texturing processes contribute to the overall functionality and usability of injection molded components.
Achieving consistent surface quality requires a combination of precise molding techniques and advanced process control. The scientific injection molding process is particularly effective in this regard. It involves determining the optimum fill speed to reduce variations in polymer flow, which directly impacts surface quality. Tools like the in-mold rheology curve help analyze viscosity changes, enabling designers to select fill speeds that ensure uniformity. Additional techniques include:
- Using high-precision molds with minimal surface roughness.
- Implementing real-time monitoring systems to control temperature and pressure during the injection process.
- Conducting regular mold maintenance to prevent wear and tear that could affect surface quality.
These methods ensure that every part meets the stringent quality standards required in medical device manufacturing.
Mold flow simulations offer significant advantages in the injection molding process. By digitally simulating how molten plastic fills a mold cavity, engineers can identify potential issues before production begins. This predictive capability allows for design optimization, reducing errors and ensuring high-quality outcomes. For instance, simulations can predict defects such as warping or air traps, enabling adjustments to the mold design and manufacturing process. These proactive measures save time and costs by eliminating the need for physical tooling modifications. Mold flow analysis also enhances efficiency by streamlining the injection process and maintaining consistent quality across all parts.
Pre-production mold flow analysis plays a critical role in identifying flaws in the injection molding design. This approach prevents costly errors and ensures smooth manufacturing operations. Key benefits include:
- Optimizing gate placement for even material flow.
- Enhancing the speed and efficiency of the molding process.
- Identifying potential problem areas, such as uneven cavity filling or tooling errors.
By addressing these issues early, manufacturers can produce high-quality parts while minimizing waste. Mold flow analysis also ensures that cavities fill evenly, reducing the likelihood of defects and improving overall product reliability.
Advanced modeling tools enhance the accuracy of mold flow analysis, ensuring precise control over the injection molding process. These tools allow experimentation with gate designs, predict shrinkage, and visualize internal stresses. The following table highlights their capabilities:
| Aspect | Description |
|---|---|
| Gating System Design | Experimentation with gate placements and sizes for optimal flow. |
| Shrinkage Prediction | Accurate predictions using material data and temperature distribution. |
| Stress and Warpage Prediction | Visualization of internal stresses and deformations to optimize design and cooling processes. |
These tools are widely used in industries such as automotive, medical devices, and aerospace. They ensure that critical components meet performance and safety standards. By combining mold flow analysis with Design for Manufacturability (DFM), manufacturers can improve mold quality, enhance production efficiency, and reduce costs.
Effective venting systems are essential for maintaining the quality of injection molded parts. These systems allow trapped air and gases to escape during the injection process, ensuring the molten material fills the mold cavity uniformly. Without proper venting, air traps can form, leading to defects such as burn marks, incomplete filling, or weak weld lines. Engineers design venting systems by incorporating small channels or gaps at strategic locations in the mold. These pathways facilitate the escape of air without compromising the structural integrity of the part. Advanced simulation tools help identify optimal venting locations, improving the overall efficiency of the molding process.
Air traps are a common issue in injection molding, often caused by inadequate venting. These traps occur when air becomes compressed within the mold cavity, preventing the molten material from filling the space completely. Proper venting eliminates this problem by providing escape routes for air and gases. This ensures uniform filling and reduces the likelihood of defects. Key engineering benchmarks validate the importance of well-designed venting systems:
- Proper venting ensures seamless flow of molten material while allowing trapped air to escape.
- Effective venting prevents air traps, ensuring uniform mold filling and defect-free parts.
By addressing air traps early in the design phase, manufacturers can enhance product quality and minimize production waste.
Proper venting plays a critical role in maintaining the integrity of injection molded parts. It prevents defects that could compromise the strength, appearance, or functionality of the final product. For example, burn marks caused by trapped gases can weaken the material and affect its durability. Venting systems also contribute to process control by ensuring consistent cavity filling and reducing internal stresses. Regular maintenance of venting channels further enhances their effectiveness, ensuring long-term reliability. By prioritizing venting in mold design, manufacturers can achieve higher quality standards and improve the overall efficiency of the injection molding process.
Regular maintenance is vital for ensuring the longevity and consistent performance of injection molds. Over time, residue buildup, wear, and minor damages can compromise the quality of molded parts and disrupt the injection process. By implementing a structured maintenance schedule, manufacturers can prevent unexpected downtimes and costly repairs. Key practices include regular cleaning to remove debris, inspections to identify early signs of wear, and lubrication to reduce friction on moving components. These measures not only extend mold life but also maintain the quality of the injection molding process.
| Maintenance Practice | Benefit |
|---|---|
| Regular cleaning | Prevents buildup that can cause wear |
| Regular inspection | Identifies signs of wear early |
| Lubrication | Reduces friction and wear on moving parts |
| Prompt repairs | Addresses issues before they worsen |
| Preventive maintenance schedule | Reduces unscheduled maintenance events |
Extending the service life of molds requires a proactive approach. Regular inspections and cleaning help avoid larger issues by addressing minor damages early. Maintaining optimal temperature control during the injection process prevents thermal degradation, ensuring consistent quality. Lubrication and rust prevention products safeguard mold components from premature wear and corrosion. For example, an electronics manufacturer reported a 20% increase in mold lifespan after implementing climate-controlled storage. Molds stored under these conditions achieved an average life expectancy of 1.2 million cycles, compared to 1 million cycles in standard storage. These practices highlight the importance of environmental and operational control in mold maintenance.
Injection molds used in medical applications face unique challenges due to stringent quality requirements. Preventing wear and tear involves maintaining precise control over the injection process and ensuring molds operate within their design specifications. Temperature fluctuations, improper lubrication, and residue buildup can lead to defects in medical-grade parts. Regular maintenance, including cleaning and lubrication, minimizes these risks. Additionally, using high-quality materials for mold construction enhances durability. By prioritizing these measures, manufacturers can produce reliable medical devices while reducing the frequency of mold replacements.
Regulatory compliance is a cornerstone of injection molding for medical devices. Adhering to FDA and ISO standards ensures that products meet stringent safety and performance requirements. These regulations guide the design and manufacturing processes, particularly in sensitive industries like pharmaceuticals. For instance, FDA regulations emphasize quality risk management to prevent contamination and maintain product safety throughout its lifecycle. Incorporating preventive actions during the initial design phase significantly reduces risks.
| Key Point | Description |
|---|---|
| Importance of FDA Regulations | Adhering to FDA regulations is crucial in the design and manufacturing processes, particularly in pharmaceuticals. |
| Quality Risk Management | Implementing quality risk-management measures is essential for preventing contamination and ensuring product safety throughout its lifecycle. |
| Preventive Actions | Incorporating preventive actions in the initial design phase can significantly reduce the risk of contamination. |
By understanding these standards, manufacturers can align their injection molding processes with global compliance requirements, ensuring consistent quality and safety.
Designing for compliance involves integrating regulatory requirements into every stage of the injection molding process. This approach ensures that molds and parts meet the necessary standards for medical applications. Engineers must consider factors such as material selection, dimensional accuracy, and process control. For example, maintaining tight tolerances and uniform wall thickness helps achieve consistent quality. Additionally, robust process control systems monitor critical parameters like temperature and pressure, ensuring compliance with regulatory benchmarks. By embedding compliance into the design phase, manufacturers can streamline production and avoid costly redesigns or regulatory penalties.
Comprehensive documentation and validation are essential for regulatory compliance in injection molding. These processes verify that the mold design and manufacturing methods meet established standards. Engineers perform a series of tests, including Design of Experiments (DoE), to optimize process parameters. Process capability studies ensure consistent production, while material verification confirms raw material quality. Quality control tests assess dimensional accuracy and physical properties of the final parts. All findings are documented in a detailed validation report, providing a clear record of compliance. This meticulous approach not only ensures regulatory adherence but also enhances product reliability and customer trust.
By prioritizing documentation and validation, manufacturers can maintain high standards in injection molding while meeting regulatory expectations.
Precision injection molding design demands meticulous attention to detail. Factors like material selection, wall thickness, and cooling systems directly influence the quality of molded parts. Integrating these tips into the injection process ensures consistent results and compliance with industry standards. Effective control over parameters like temperature and pressure enhances product reliability. By prioritizing precision and quality, manufacturers can achieve success in medical device manufacturing and deliver dependable products to the market.
The most critical factor is precision. Injection molding for medical devices requires tight tolerances to ensure parts meet regulatory standards. Precision in the design and process guarantees consistent quality and functionality, which are essential for patient safety.
Regular mold maintenance ensures consistent quality and extends the mold's lifespan. Cleaning, lubrication, and inspections prevent defects and reduce downtime. A well-maintained mold improves the injection process by maintaining control over critical parameters like temperature and pressure.
Uniform wall thickness ensures even cooling and material flow during the injection process. This consistency prevents defects like warping and sink marks. It also enhances the strength and quality of the final product, making it suitable for demanding applications.
Efficient cooling systems reduce cycle times and ensure uniform cooling, which prevents defects like residual stress and warping. Proper cooling control enhances the quality of molded parts and increases production efficiency, making the manufacturing process more reliable.
Mold flow analysis identifies potential issues like air traps or uneven filling before production begins. This analysis optimizes the design and process, ensuring high-quality outcomes. It also reduces waste and improves control over the injection molding process.