
Cleanroom standards play a vital role in medical device manufacturing. These controlled environments help you maintain sterility and prevent contamination. When you follow these standards, you ensure that medical devices meet strict safety and quality requirements. Clean room injection molding, for example, allows you to create precise components with minimal risk of impurities. By maintaining cleanroom protocols, you can improve product reliability and protect patient health.
Cleanroom standards are essential for maintaining controlled environments where contamination risks are minimized. These standards define the protocols and requirements for air cleanliness, particle control, and environmental monitoring. By adhering to clean room standards, you can ensure that medical devices are manufactured in a sterile and controlled setting. This reduces the risk of contamination and enhances the overall quality of the products.
Cleanrooms are designed to control airborne particles, temperature, humidity, and pressure. For example, HEPA filters remove 99.97% of particles as small as 0.3 microns, while ULPA filters achieve even higher efficiency, capturing 99.999% of particles up to 100 nanometers. These filtration systems play a critical role in maintaining the cleanliness of the environment.
Two key regulations guide cleanroom practices in medical device manufacturing: ISO 14644 and ISO 13485. ISO 14644 focuses on air cleanliness and provides a framework for controlling airborne particles in cleanrooms. It establishes methodologies for monitoring and maintaining biocontaminant levels. For instance, ISO 14644 classifies cleanrooms based on the concentration of particles per cubic meter, ranging from ISO Class 1 (the cleanest) to ISO Class 9.
ISO 13485, on the other hand, emphasizes quality management systems for medical devices. It ensures that your manufacturing processes meet regulatory requirements and produce safe, high-quality products. By following ISO 13485, you can demonstrate compliance with global standards and build trust with customers and regulatory bodies.
| Standard | Focus Area |
|---|---|
| ISO 14644 | Methodology for biocontaminant removal |
| ISO 13485 | Quality management for medical devices |
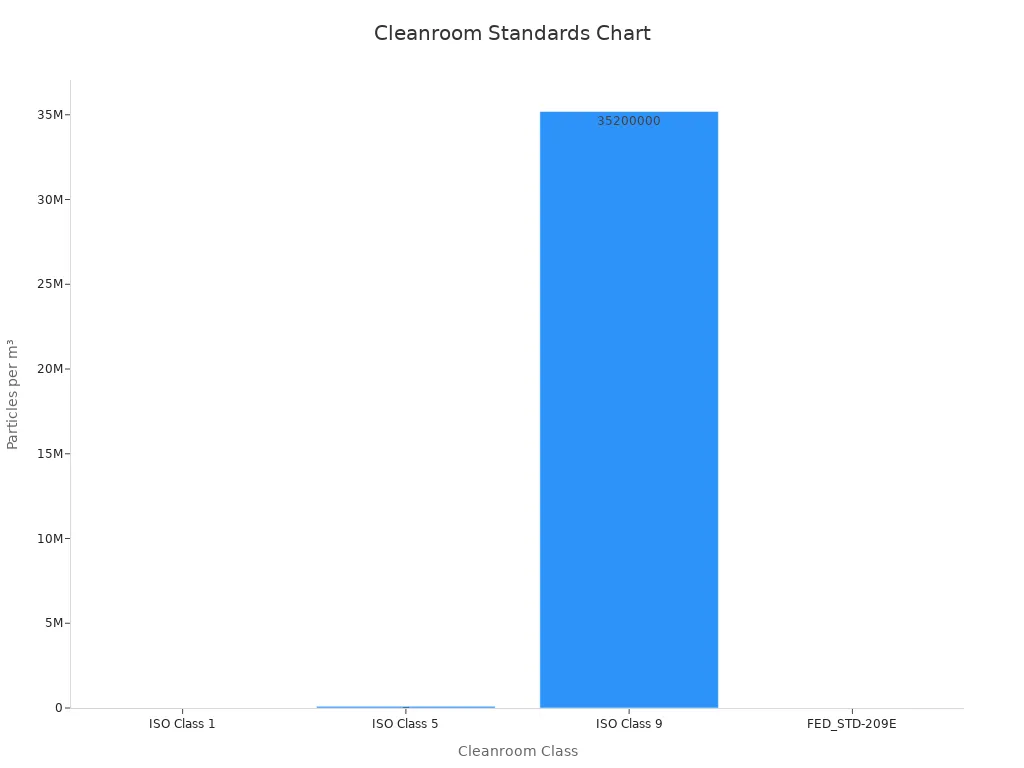
Cleanroom classifications are based on the concentration of airborne particles within a specific size range. These classifications help you determine the level of cleanliness required for your manufacturing processes. For example, ISO Class 1 cleanrooms allow only 10 particles larger than 0.1 microns per cubic meter, while ISO Class 9 cleanrooms are comparable to normal room air. Most medical device manufacturing cleanrooms fall under ISO Class 7 or ISO Class 8.
| Cleanroom Class | Particle Size (µm) | Particles per m³ |
|---|---|---|
| ISO Class 1 | 0.1 | 10 |
| ISO Class 5 | 0.1 | 100,000 |
| ISO Class 9 | 0.5 | 35,200,000 |
Maintaining air quality control is critical for meeting these classifications. Advanced filtration systems, such as HEPA and ULPA filters, ensure that cleanrooms meet the required standards. For instance, a Class 7 cleanroom can have a maximum particle concentration of 352,000 particles per cubic meter. Regular monitoring and validation of air quality help you maintain compliance with cleanroom classifications and ensure the integrity of your manufacturing processes.

Gowning and hygiene procedures are essential for maintaining the cleanliness of a cleanroom. You must follow strict protocols to prevent contamination during clean room injection molding. Proper gowning involves wearing specialized garments, such as coveralls, gloves, masks, and shoe covers, designed to minimize the release of particles from your body. These garments act as a barrier, ensuring that contaminants like skin flakes, hair, and dust do not enter the cleanroom environment.
Before entering the cleanroom, you should perform thorough cleaning and disinfection of your hands. This step reduces the risk of introducing microbes or particles into the controlled environment. Gowning must occur in a designated area, often called a gowning room, where you can put on cleanroom-approved attire in a specific sequence. For example, you might start with shoe covers, followed by gloves, and then a full-body suit. This sequence ensures maximum hygiene and minimizes contamination risks.
Hygiene protocols extend beyond gowning. Regular cleaning and disinfection of surfaces, tools, and equipment are necessary to maintain a sterile environment. You should also adhere to strict behavioral guidelines, such as avoiding unnecessary movements and speaking softly, to reduce particle generation. These practices ensure that the cleanroom remains compliant with cleanliness standards, enhancing the quality of medical devices produced.
Proper material handling and storage are critical for maintaining the integrity of materials used in clean room injection molding. You must ensure that all materials entering the cleanroom are thoroughly cleaned and sterilized. This step prevents the introduction of contaminants that could compromise the manufacturing process. Filters and traps are often used to eliminate airborne particles during material transfer, ensuring a safe and clean production environment.
Once inside the cleanroom, materials should be stored in designated areas that meet cleanliness standards. For example, sealed containers or cabinets can protect materials from exposure to airborne particles. You should also label and organize materials to facilitate traceability and prevent cross-contamination. Regular inspections of storage areas help maintain compliance with cleanroom protocols.
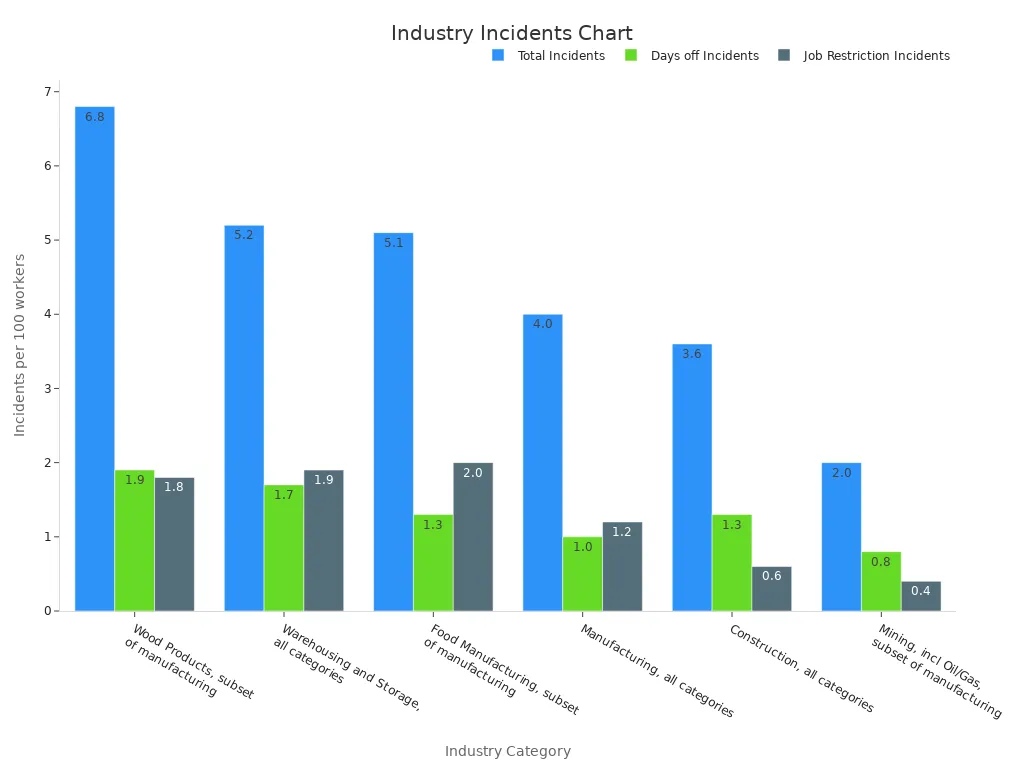
Statistical data highlights the importance of efficient material handling and storage procedures. For instance, industries with stringent handling protocols, such as manufacturing and warehousing, report lower incident rates compared to others.
| Industry Category | Total Incidents, per 100 workers | Incidents w/days off, per 100 workers | Incidents w/job transfers or restrictions, per 100 workers |
|---|---|---|---|
| Manufacturing, all categories | 4.0 | 1.0 | 1.2 |
| Warehousing and Storage, all categories | 5.2 | 1.7 | 1.9 |
| Food Manufacturing, subset of manufacturing | 5.1 | 1.3 | 2.0 |
By implementing robust material handling and storage procedures, you can reduce contamination risks, improve efficiency, and ensure the quality of medical devices.
Equipment maintenance and validation are vital for ensuring the reliability of cleanroom operations. You must regularly inspect and maintain equipment to prevent malfunctions that could compromise cleanliness. Validation processes confirm that equipment and systems meet required specifications and perform as intended under normal operating conditions.
The validation process typically includes several steps:
- Design Qualification (DQ): Confirms that the cleanroom design meets industry standards.
- Installation Qualification (IQ): Verifies that equipment is installed correctly.
- Operational Qualification (OQ): Tests equipment under normal conditions to ensure proper functionality.
- Performance Qualification (PQ): Assesses long-term performance to maintain cleanliness standards.
Ongoing environmental monitoring is another critical aspect of validation. You should regularly check air quality, temperature, humidity, and particulate levels to ensure compliance with cleanroom standards. Microbial testing can also identify potential contamination sources, allowing you to take corrective actions promptly.
Comprehensive documentation of maintenance and validation activities is essential for regulatory compliance. Detailed records demonstrate your commitment to quality and provide evidence of adherence to industry standards. By prioritizing equipment maintenance and validation, you can enhance the reliability of your cleanroom operations and produce high-quality medical devices.
Traceability is a cornerstone of the quality control process in medical device manufacturing. It allows you to track every component and material used in production, ensuring compliance with regulatory standards. By implementing robust traceability systems, you can identify and address potential issues before they escalate. For example, environmental monitoring in cleanroom environments helps detect contamination sources, such as air or surface particles, and ensures product safety.
A case study revealed that comprehensive sampling of cleanroom environments, including air, surfaces, and utilities like compressed air, significantly improved traceability. This approach reduced laboratory errors and minimized manufacturing failures.
Traceability also supports validation and auditing processes. Detailed records of materials, equipment, and personnel involved in production provide a clear audit trail. This transparency not only enhances compliance but also builds trust with regulatory bodies and customers.
Maintaining optimal conditions in cleanroom environments is essential for ensuring product quality and regulatory compliance. You can achieve this by monitoring key metrics, such as airborne particles, temperature, humidity, and microbial contamination.
| Measurement Metric | Description |
|---|---|
| Airborne Particle Monitoring | Continuous sampling and quantification of airborne particles to maintain cleanliness standards. |
| Environmental Parameter Monitoring | Monitoring of temperature, humidity, and pressure to ensure optimal cleanroom conditions. |
| Microbiological Monitoring | Detection and analysis of microbial contamination risks through automated sampling methods. |
| Gowning Room Monitoring | Ensuring compliance with gowning protocols to prevent contamination from personnel. |
Regular monitoring helps you identify trends and deviations that could compromise the cleanroom's integrity. For instance, statistical process control (SPC) methods, such as control charts, allow you to track variability and maintain consistent quality. By addressing issues promptly, you can prevent costly production delays and ensure compliance with regulatory standards.
Accurate documentation is vital for meeting regulatory compliance requirements. It serves as evidence during auditing and inspections, safeguarding your operations against fines and non-compliance risks. Comprehensive records demonstrate your commitment to quality and adherence to industry standards.
Key types of documentation include:
- Cleaning logs that detail cleaning activities, personnel, and agents used.
- Equipment calibration records to ensure proper functioning of tools.
- Batch records that document the entire manufacturing process for traceability.
Additionally, maintaining design documents, qualification protocols, and environmental monitoring logs strengthens your compliance efforts. These records support validation and auditing processes, ensuring your cleanroom operations meet regulatory expectations. By prioritizing documentation and traceability, you can enhance product safety and build trust with stakeholders.

Cleanroom standards play a vital role in reducing contamination risks during medical device manufacturing. By maintaining controlled environments, you can prevent airborne particles, microbes, and other contaminants from compromising product integrity. Specialized filtration systems, such as HEPA filters, ensure that cleanrooms meet stringent cleanliness requirements. These systems remove nearly all particles, creating a sterile environment for production.
The growing adoption of medical devices in healthcare settings highlights the importance of cleanroom technology. Cleanrooms ensure hygiene and reliability, which are essential for patient safety. Energy-efficient designs further enhance cleanroom operations, helping manufacturers meet sustainability goals while maintaining contamination control.
Cleanrooms are indispensable for ensuring product safety and quality. They significantly reduce contamination risks, making them a cornerstone of medical device manufacturing.
Cleanroom practices directly improve the reliability and performance of medical devices. Stringent environmental controls, such as temperature and humidity regulation, maintain optimal conditions for production. These measures reduce the risk of defects and ensure consistent product quality. Continuous monitoring allows you to detect deviations promptly, preventing costly errors.
| Parameter | Importance in Cleanroom Practices |
|---|---|
| Particle Count | Prevents contamination during production processes. |
| Temperature | Maintains optimal conditions for product integrity. |
| Humidity | Reduces the risk of microbial growth and contamination. |
| Pressure | Ensures compliance with regulatory standards. |
In industries like pharmaceuticals, maintaining a sterile environment is critical. Microbial contamination can compromise medication integrity, just as particle contamination can affect the performance of electronic components. Cleanroom standards ensure that medical devices meet high reliability and performance benchmarks.
Adhering to cleanroom standards helps you achieve compliance with regulatory requirements and industry benchmarks. These standards ensure that your manufacturing processes align with global expectations for safety and quality. For example, ISO 13485 emphasizes quality management systems, while ISO 14644 focuses on air cleanliness. By following these guidelines, you can demonstrate your commitment to producing safe and reliable medical devices.
The cleanroom technology market continues to grow, driven by the rising demand for contamination control. Manufacturers increasingly prioritize energy-efficient designs to meet both financial and environmental objectives. These advancements not only enhance compliance but also improve operational efficiency. By implementing cleanroom standards, you can build trust with regulatory bodies and customers, ensuring long-term success in medical device manufacturing.
Cleanroom standards are essential for producing safe and reliable medical devices. By following these standards, you can maintain high-quality manufacturing processes and minimize contamination risks. This ensures that your products meet strict regulatory requirements and perform as intended.
Tip: Adhering to cleanroom practices not only improves efficiency but also builds trust with customers and regulatory bodies.
When you prioritize cleanroom protocols, you create a foundation for success in medical device manufacturing. These practices help you achieve compliance, enhance product quality, and protect patient health.
Cleanroom standards help you maintain a controlled environment by reducing contamination risks. They ensure that medical devices meet strict safety and quality requirements, protecting patient health and complying with regulatory guidelines.
Cleanroom classifications define the level of air cleanliness required for production. For example, ISO Class 7 cleanrooms allow fewer particles than ISO Class 8. These classifications help you determine the necessary controls to maintain product quality.
Gowning minimizes contamination risks by preventing particles from your body, like skin flakes or hair, from entering the cleanroom. Proper gowning ensures a sterile environment, which is critical for producing high-quality medical devices.
You should validate cleanroom equipment regularly, typically during installation, operation, and performance checks. Ongoing validation ensures that equipment functions correctly and meets cleanliness standards, reducing the risk of contamination.
Monitoring cleanroom conditions helps you maintain optimal air quality, temperature, and humidity. This ensures compliance with standards, prevents contamination, and enhances the reliability of medical devices.
Tip: Regular monitoring can also identify potential issues early, saving time and costs in the long run.