
You achieved a remarkable milestone by delivering 50,000 medical device housings in just six weeks. Leveraging technology and advanced manufacturing, you ensured faster production without compromising quality. Process optimization played a vital role, reducing overdue repairs by 66% and defect rates to just 0.05% per instrument. This success highlights your dedication to transforming healthcare with precision and speed.

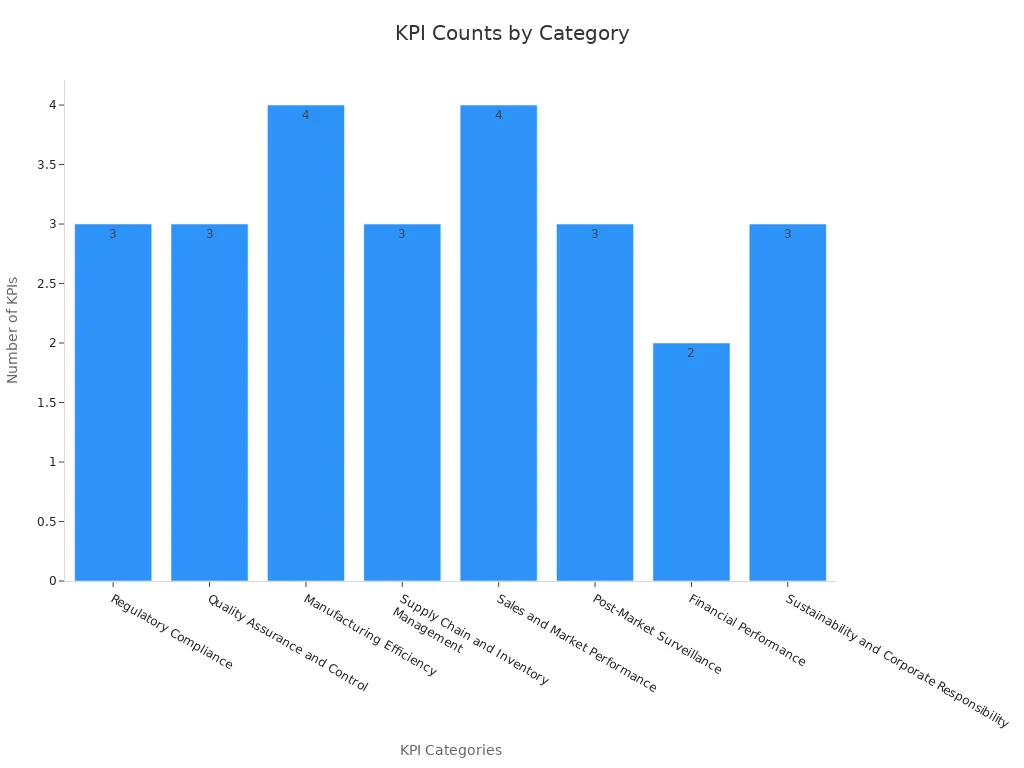
Meeting industry standards in medical manufacturing requires precision and consistency. You must adhere to strict guidelines to ensure patient safety and device reliability. Metrics such as First Pass Yield (FPY) and Defects Per Unit (DPU) help measure production quality. For example, FPY tracks the percentage of devices that pass through manufacturing without rework, while DPU highlights potential quality issues in each device. These indicators ensure that every medical device meets the highest standards before reaching healthcare services.
Maintaining these standards becomes even more critical when working under tight deadlines. Accelerated timelines can pressure you to balance speed with quality. Concerns often arise about the thoroughness of assessments and the reliability of data. For instance, compressed schedules may limit stakeholder engagement, potentially compromising the evaluation process. Despite these challenges, you must prioritize quality to maintain trust in healthcare services.
| KPI | Description |
|---|---|
| First Pass Yield (FPY) | Measures the percentage of products passing through manufacturing without needing rework. |
| Defects Per Unit (DPU) | Highlights potential quality issues in each device produced. |
| Overall Equipment Effectiveness (OEE) | Assesses production machinery performance in terms of speed, quality, and uptime. |
| Customer Complaint Rate | Gauges customer satisfaction regarding product quality or performance. |
The initial stages of production often reveal hidden challenges. Data preparation is one of the most demanding phases. Many operational systems are not designed for data recording, making it difficult to generate data-driven decisions. When you test data, issues such as poor quality or incomplete records can disrupt production. These problems emphasize the importance of addressing data challenges early to avoid delays.
Supply chain management also faces hurdles during the pilot phase. Traditional methods like safety stocks and stock alerts may not align with modern probabilistic forecasts. This shift can create uncertainty, testing both the supply chain logic and the technology. For example, automated decisions must work without manual corrections, which can be a significant challenge. Overcoming these obstacles requires a focus on innovation and adaptability to ensure smooth production and delivery of medical devices.
You can achieve remarkable production speeds by adopting advanced manufacturing techniques and automation. These methods integrate cutting-edge technology to streamline processes and reduce human intervention. For example, continuous manufacturing combines multiple steps into a single system. This approach has proven effective in the healthcare sector, enabling faster production of critical items like influenza vaccines. By responding quickly to market demands, you can ensure that patients receive timely care.
Automation also plays a vital role in improving efficiency. Robotic process automation (RPA) reduces downtime and accelerates production cycles. This allows you to meet tight deadlines without compromising quality. Additionally, digital twins—virtual replicas of physical systems—enable real-time monitoring and optimization of production processes. This technology minimizes errors and ensures that medical devices meet the highest standards.
| Aspect | Advanced Manufacturing | Traditional Manufacturing |
|---|---|---|
| Technology Adoption | Integrates latest technologies (AI, IoT) | Relies on manual and semi-automated processes |
| Process Automation | High automation, reducing human intervention | Dependent on manual labor |
| Data Utilization | Data-driven insights for real-time monitoring | Lacks integrated real-time data |
| Production Speed | Faster production cycles due to automation | Slower, labor-intensive processes |
By leveraging these advancements, you can enhance production speed, improve patient outcomes, and maintain the quality of medical devices.
Optimizing workflows is essential for achieving efficiency in medical manufacturing. Streamlined processes eliminate bottlenecks and reduce delays, ensuring that you meet delivery deadlines. For instance, Total Quality Management (TQM) emphasizes continuous improvement and data-driven decision-making. This methodology enhances team collaboration and optimizes processes, leading to better productivity.
Localizing production facilities can also improve efficiency. Nipro Medical Corporation, for example, established a manufacturing facility in North Carolina to reduce transportation costs and respond quickly to customer needs. This strategy not only accelerates delivery times but also strengthens supply chain resilience.
Tip: Use data analytics to identify inefficiencies in your workflow. By addressing these issues, you can improve productivity and ensure that medical devices reach patients faster.
Effective team collaboration is the backbone of rapid production. When team members communicate frequently and share knowledge, they can address challenges more efficiently. Real-time collaboration tools enable seamless teamwork across multiple locations, reducing delays and ensuring project success.
Advanced Product Data Management (PDM) systems further enhance collaboration. These systems eliminate bottlenecks and guarantee that everyone stays aligned with project goals. Metrics like communication frequency and project completion rates highlight the importance of teamwork in achieving accelerated production.
| Metric | Description |
|---|---|
| Communication Frequency | Measures how often team members communicate, impacting engagement and clarity. |
| Project Completion Rates | Indicates the efficiency of collaboration by tracking how quickly projects are completed. |
| Knowledge Sharing | Enhances productivity and camaraderie among employees, fostering a collaborative environment. |
| Cross-Functional Collaboration | Reflects the integration of different departments, improving overall communication and teamwork. |
| Feedback and Recognition | Essential for maintaining motivation and ensuring projects stay on track through continuous input. |
By fostering a culture of collaboration, you can accelerate production timelines and deliver high-quality medical devices that improve patient outcomes.
Achieving rapid production of medical devices requires careful planning and execution. You must track key milestones to ensure progress aligns with deadlines. For example, completing documentation for Phase I audits is a critical step. This milestone ensures compliance with regulatory standards and prepares the groundwork for subsequent phases. By Q1 2024, 79% of this documentation was completed, demonstrating significant progress.
Another milestone involves in-house verification testing of prototypes. For the Huygens™ Catheter, this testing phase ensures the device meets performance and safety standards. Completing assembly and testing of electronic subsystems further validates the device's functionality. These milestones highlight the importance of structured timelines in medical device innovations.
| Milestone Description | Percentage/Count | Deadline |
|---|---|---|
| Completion of documentation for Phase I audit | 79% | Q1/2024 |
| Number of documents established related to design, production, tracing, and safety | 36 | N/A |
| Complete in-house verification testing of the Huygens™ Catheter prototype | N/A | Q1/2024 |
| Complete assembly and testing of the Huygens™ Catheter electronic subsystems | N/A | Q1/2024 |
| Prepare documentation for Phase I and II ISO 13485 Certification audit | N/A | Q1/2024 |
| Prepare materials for non-GLP Animal Study | N/A | Q2/2024 |
| Prepare materials for safety certification audit | N/A | Q2/2024 |
| Prepare materials for second phase testing of the Huygens™ Catheter | N/A | Q2/2024 |
Note: Tracking milestones ensures that every phase of production remains on schedule, reducing delays and improving outcomes for patients.
Maintaining quality during accelerated production is essential. You can use statistical tools like control charts and run charts to monitor process stability. These tools help identify variations that could impact the quality of medical devices. For example, control charts provide a visual representation of process performance over time, allowing you to detect anomalies early.
Design of Experiments (DOE) is another effective method. This structured approach helps you understand how multiple variables affect quality. By optimizing these variables, you can enhance the reliability of medical devices. Additionally, material testing and performance data play a crucial role in ensuring compliance with industry standards.
| Statistical Measure | Description |
|---|---|
| Control Charts | Graphical tools used to monitor process stability and detect variations over time. |
| Run Charts | Visual representations of data trends over time, helping to identify patterns and anomalies. |
| Design of Experiments | A structured approach to experimentation that helps in understanding the effects of multiple variables on quality. |
| Key Element | Importance |
|---|---|
| Material testing results | Provides objective evidence that the product meets performance requirements and specifications. |
| Performance test data | Verifies that the product performs as intended under various conditions. |
| Environmental testing results | Ensures compliance with environmental specifications. |
| Accelerated life testing (HALT) | Validates product durability under stress conditions. |
| Third-party lab certifications | Adds credibility to the testing results and compliance claims. |
Tip: Use statistical methods to continuously monitor and improve production processes. This ensures that every device meets the highest quality standards.
The final delivery of medical devices marks the culmination of months of effort. By adhering to strict timelines and quality standards, you can ensure that these devices reach healthcare providers on time. This rapid delivery has a profound impact on patient outcomes. For instance, faster production cycles enable healthcare professionals to address urgent needs, such as during a pandemic or medical crisis.
Statistics play a vital role in measuring the impact of these innovations. Healthcare providers use statistical tools to assess performance and establish benchmarks. These methods help improve decision-making and enhance care quality. For example, statistical analysis can determine the optimal combination of goods and services to minimize costs and maximize outcomes.
By delivering high-quality medical devices quickly, you contribute to better healthcare outcomes. Patients benefit from timely access to innovative technology, improving their quality of life and overall health.
Callout: Rapid production and delivery of medical devices save lives. By leveraging advanced technology and data-driven processes, you can make a lasting impact on healthcare.
You achieved rapid delivery of 50,000 medical housings by combining advanced manufacturing, efficient workflows, and teamwork. These strategies ensured quality while meeting tight deadlines. By prioritizing data-driven decisions, you improved healthcare outcomes and patient care. Explore how your expertise can transform medical production and support healthcare providers in delivering better solutions.
Advanced manufacturing techniques improve production speed and quality. They help you meet healthcare delivery demands while ensuring compliance with strict industry standards.
Data enables real-time monitoring and decision-making. It helps you identify inefficiencies, optimize workflows, and maintain high-quality standards during rapid production cycles.
Yes, telehealth technology relies on timely access to innovative devices. Faster production ensures healthcare providers can deliver remote care effectively, improving patient outcomes.